Center for Global Bioethics
The CREEi Center for Global Bioethics (CGB) was established in WINDREF by the Caribbean Research Ethics Education initiative (CREEi) in 2021, supported by the NIH Fogarty International Center Award # R25TW009731 (2014-2026). The CGB serves as a center of excellence in research ethics and global bioethics.
Global bioethics illuminates social and physical environments and ecosystems that influence health and wellbeing; it can be used to navigate values, norms, policies, and responsibilities across distance and time (VR Potter. Global Bioethics. 1981. Michigan State University Press)
CGB Mission
The Center for Global Bioethics (CGB) advances ethically informed, environmentally sensitive, and equitable education and analytic research about intersections between health, health priorities, and environments.
CGB Goals
- To advance global bioethics through collaborative partnerships in education and research.
- To curate and make available resources for research ethics and global bioethics that are regionally sensitive and, when possible, in both English and Spanish languages.
- To investigate and better understand the dependence of health on environments and ecosystems.
- To promote public trust in science and healthcare.
CBG Executive, Advisors, and Faculty
- Director Cheryl C Macpherson PHD
- Deputy Director Kareem Coomansingh, MPH, MScB
- Administrative Associate Naomi Whyte
Advisory Board
- Shereen Cox, Pharmacist, Ethics lecturer, Doctoral Research Fellow, University of Oslo, Norway
- Elizabeth Heitman, Program in Ethics in Science and Medicine, University of Texas Southwestern Medical Center, USA
- Ruth Macklin, Distinguished University Professor Emerita, Albert Einstein College of Medicine, Bronx, NY, USA
- Cristina Richie, Lecturer, Philosophy and Ethics of Technology, Delft University of Technology, Delft, Netherland
- Carla Saenz, Knowledge Management, Bioethics and Research, Pan American Health Organization (PAHO), USA
- Calum Macpherson, Director, WINDREF
- Trevor Noel, Deputy Director, WINDREF
Faculty Consultants
- Derrick Aarons, PhD, MBBS (Jamaica)
- Jonathan Ashcroft, MD, PhD (England)
- Paul J Cummins, PhD (USA)
- Bernardo Garcia, PhD, MScB (Mexico)
- Nicholas Jennings, PhD (Trinidad & Tobago)
- Sharmella Roopchand Martin, DPT, MScB (Jamaica)
About Caribbean Research Ethics Education initiative (CREEi)
From 2020-2024, CREEi educated 45 fellows in a two-year Master’s in Bioethics program (MScB) developed by collaborators from St George’s University (SGU) in Grenada, Universidad Autónoma de Querétaro (UAQ) in Mexico, and Clarkson University in the USA. Since 2024, SGU and UAQ have offered two-year master’s degree programs patterned on CREEi’s curriculum.
Graduates of the CREEi MScB program are equipped to serve as research ethicists and educators, advocates for protection of human and animal subjects in research, and institutional and national leaders in their home countries. Many have contributed to curricular and course design and teaching. CREEi’s curriculum has advanced Caribbean capacity for the responsible conduct of research, research ethics review, and research institutions and policy.
About 33% of CREEi MScB graduates are from English-speaking low- and middle-income countries (LMIC) of the Caribbean basin with 66% from Spanish-speaking LMICs of the region. The provision of live interpretation for some curricular and course activities, and the use of artificial intelligence translations for others, made it possible to educate English- and Spanish-speakers simultaneously in the same courses and for them to communicate with faculty and each other in both languages even without being bilingual. This cross-cultural and international integration enriched their learning and expanded their regional research ethics and bioethics network.
From 2014-2019, CREEi trained 80 professionals from 17 LMICs of the Caribbean basin in a one-year certificate program. This program ran in parallel English-speaking and Spanish-speaking arms of roughly equal cohort sizes.
CREEi Aims
- To provide a culturally relevant knowledge base and skill set in research ethics that will allow fellows from low- and middle-income countries (LMICs) in the Caribbean to function as independent research ethicists in their home countries and institutions through a new 2-year Masters degree program using our previous hybrid model (onsite and online) and offering new opportunities for research and publication.
- To prepare fellows to act as researchers, research ethics educators, leaders in research ethics committees, and as research and ethics policy consultants in their home countries and institutions.
- To prepare fellows to facilitate institutional change of ethical policy and practices in research.
- To develop and maintain centers of excellence in bioethics and research ethics at St. George’s University (SGU) and the Universidad Autónoma de Querétaro (UAQ).
- To develop and sustain a bilingual research ethics network in the Caribbean that expands the existing CREEi alumni network.
- To develop additional research ethics capacity in the Caribbean by developing and delivering a new series of annual workshops in research ethics offered by CREEi fellows and faculty for researchers, ethics committee members, and other stakeholders in LMIC of the Caribbean basin.
These aims were fulfilled through the collaborative design and delivery of the CREEi MScB curriculum and courses, and through support from two NIH-FIC administrative supplements to CREEi for professional development opportunities for its graduates.

CREEi Curriculum and Courses
The two-year CREEi Master’s degree program was collaboratively designed by the CREEi Principal Investigator and Co-Investigators in consultation with faculty, Advisory Board members, and graduates of its one-year certificate program. It is equivalent to 34 graduate credits in the semester system used by many graduate programs in North America.
The curriculum upholds internationally recognized standards and guidance for research ethics and uses a hybrid onsite-online approach that was highly participatory, interactive, and context-specific. Bringing together fellows and faculty for intensive onsite courses at the beginning (Proseminar) and the end (Capstone) of program fostered and sustained camaraderie and nurtured personal and professional relationships that ground their regional research ethics network and link them with regional bioethics bodies such as the Bioethics Society of the English-speaking Caribbean (BSEC) and others in Spanish-speaking LMICs.
Additional information is below and the course syllabi, assignment instructions, and readings are available BELOW in both English and Spanish languages.

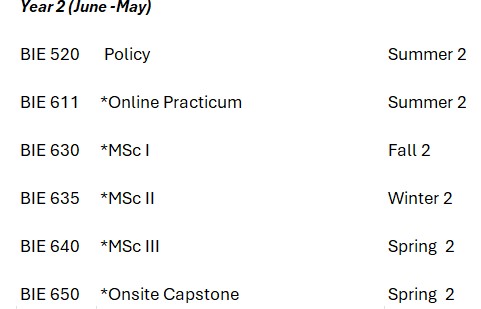
* These courses* deliver activities and mentoring that support fellows in designing, conducting, and publishing their masters research, or in conducting a policy or pedagogy project relevant to their career interests. For research, normative rather than empirical methods were encouraged.
International Research introduces scientific disciplines and research methods including biomedical, animal, biotechnology, social sciences, public health; and international, regional, and other contextual influences on research priorities, choice of research methods, and risk-benefit ratios. While learning about these, fellows formulate the research question which becomes the basis for their master’s research, begin to design a protocol to answer it, and identify the mentor who will also serve as their research supervisor throughout their master’s degree.
The Onsite Practicum provides practical experiences in i) research ethics reviews and audit; ii) communication skills and pedagogy that they use to co-teach an incoming cohort; iii) refining their research question and protocol; and iv) developing content for the local stakeholder workshop that they will contribute to teaching in their Capstone course.
During the Online Practicum fellows conduct their literature review, and produce a first draft of the introduction and methods of their thesis. Where feasible, the thesis is written in the form of a paper for publication in a mainstream peer reviewed journal or for an institutional policymaking body or curriculum committee.
The three online MSc courses include structured activities that facilitate fellows in conducting their research, policy, or pedagogy project for their master’s thesis. During MSc I fellows finalize the entire outline for their paper or project, begin normative analysis or data collection using secondary data, begin data analysis once collection is complete, and edit their introduction and methods sections. During MSc II data collection and analysis are completed and results are drafted. During MSc III conclusions (and recommendations if any) are drafted; the work is edited and prepared for submission to a journal or relevant institutional body, and for grading as the final Capstone Project. Each fellow defends their thesis during the onsite Capstone course when they also deliver the workshop for local stakeholders.
Open Access CREEi MScB Course Materials
Linked through each course bulleted below
Here we provide open access to course materials from the CREEi two-year Master’s in Bioethics degree program (MScB) including curricular structure and course syllabi, a majority of activities, instructions, grading rubrics, instructional videos, and more in both English and Spanish languages. Of note is a course on the responsible conduct of research (RCR) that attained certification from Quality Matters. Not all the materials are available here because the courses were designed to be preserved through backup cartridges of the courses from the learning management system that hosted our courses. For faithful replication of the courses content, we recommend that you specially request a backup cartridge. The cartridges are available as a backup cartridge for Moodle, which was the learning management system (LMS) we used, and in a general back that can be imported into other LMS like Blackboard or Canvas.
All materials here are made available by CREEi and the Center for Global Bioethics as a resource for those developing research ethics education programs in low- and middle-income countries in any region or language, and for those teaching in such courses or programs. We ask that users do not use these materials for artificial intelligence training or commercial purposes. For queries on the provided materials or requests, please send an email to: creei.materials@gmail.com.
Please cite these materials as below when using or adapting them.
Caribbean Research Ethics Education Initiative. “CREEi Open Access Course Materials.” WINDREF Center for Global Bioethics. 2025. https://www.windref.gd/center-for-global-bioethics/
